Seraph 100 Microbind Affinity Blood Filter
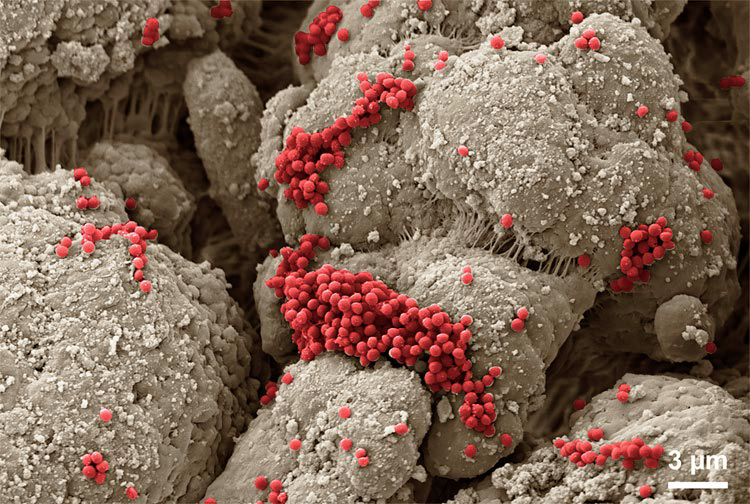
Scanning electron microscopy of S. aureus Newman attached to heparin-coated beads1
1. Seffer et al, Staphylococcus aureus Binding to Seraph® 100 Microbind® Affinity Filter: Effects of Surface Protein Expression and Treatment Duration, PLOS One, 2023;18(3):1-14
Our Technology
ExThera’s proprietary Seraph® 100 Microbind® Affinity Blood Filter (Seraph 100) is the first and only pathogen adsorption therapy available under Emergency Use Authorization (EUA) in the United States of America. The clinical experience of Seraph 100 in COVID-19 has been reported in over 50 publications.
Why use the Seraph 100?
The Seraph 100’s broad-spectrum ability reduces COVID-19 in the bloodstream and may improve patient trajectory, even prior to mechanical ventilation.
Funded Collaboration Courtesy of the United States Defense Advanced Research Programs Agency (DARPA)
ExThera’s technology was evaluated in a collaborative research program with Battelle Memorial Institute with the goal of developing a dialysis-like therapeutic (DLT) intrinsic separation device to treat sepsis in wounded warriors—all funded by a grant from DARPA.
ExThera had the only device evaluated in the final phase of the competitive program after other technologies were “down selected” because they were unable to meet the program’s goals. Because of ExThera Medical’s participation, DARPA considers the DLT program a success.
MM0002 Rev B
